An all-inclusive system converts wastewater to consumable water.
First of Four Parts
Editors Note: This is the first of a four-part series that provides a comprehensive evaluation of controlling collection system odor and corrosion using onsite oxygen and ozone generation. This series will cover the biochemistry of odor generation in the collection system. It will also provide background on oxygen and ozone chemistry that is important to understanding how these gases work.
Sewage collection systems have long been subjected to issues of odor and corrosion, which is understandable given the nature of what they convey. Sewage odor, the rotten egg smell almost everyone has experienced, is a localized and political issue and is the driving force behind implementing controls in sewage collection systems.
Corrosion, however, is the issue with the greatest potential for environmental harm and real systemic and economic damage—including burst pipes, lift station degradation and other equipment and system failure. Failures of this type require the repair and replacement of collection system materials and equipment and have the potential to expose the environment to unpredictable releases of hazardous waste that are difficult to contain or recover. According to the ASCE’s 2009 Infrastructure Report Card [ASCE, 2009], where a grade of “D-” was given:
“Aging systems discharge billions of gallons of untreated wastewater into U.S. surface waters each year. The Environmental Protection Agency estimates that the nation must invest $390 billion over the next 20 years to update or replace existing systems and build new ones to meet increasing demand.”
Hydrogen Sulfide
A major contributor to odor and corrosion is hydrogen sulfide (H2S) and its associated compounds. All human waste—and subsequently, all wastewater—contain sulfur compounds, which provide the molecular basis for the generation of hydrogen sulfide. Hydrogen sulfide arises from the combination of anaerobic conditions and the presence of sulfites and sulfates in conjunction with the colonies of microorganisms present on the inner walls of all collection systems, referred to as the slime layer.
Sulfate reducing bacteria (SRB) will use these compounds in the absence of free oxygen (O2) for metabolism. These bacteria do not actually use the sulfur component, and it is available to react with water, specifically free protons (H+), which results in the generation of hydrogen sulfide. This generation pathway can be represented by the following balanced reactions [Matthews, 2010]:
(1) SO42- → S2-
(2) S2- + H2O ↔ HS- + OH-
(3) HS- + H2O ↔ H2S (aq) + OH-
(4) H2S (aq) ↔ H2S (g)
Following its generation, hydrogen sulfide can be released into the atmosphere (see Equation 4) and find its way to receptors through manholes or other junctions of the atmosphere and collection system, at which point it is an odor concern. Hydrogen sulfide is a colorless gas that has a characteristic rotten egg odor, is highly toxic and is very corrosive to certain metals. It is heavier than air, meaning it can accumulate in wells, manholes and other similar locations that do not have good ventilation. Its effect on humans, at varying concentrations relative to ambient air, is shown in Table 1 [EPA, 1985].
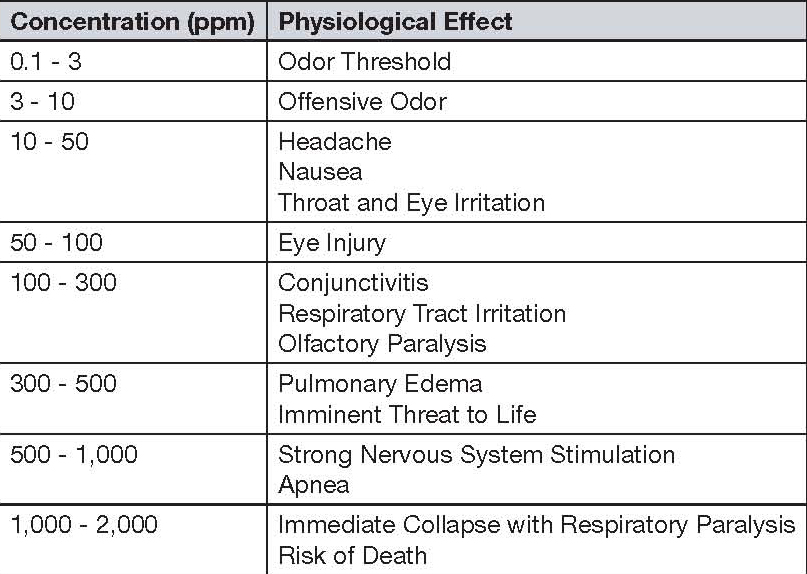 |
| Table 1. Hydrogen sulfide health effects at varying concentrations |
Hydrogen sulfide becomes a corrosion issue when it comes into contact with moist concrete or steel, among other metals, in the presence of oxygen, even at very low gaseous concentrations. Conditions such as these are fairly common in the head space of some pipes, as well as at manholes, lift stations and other areas in which the collection system is provided with easy access to atmospheric oxygen. Bacteria in these areas are able to convert the H2S into sulfuric acid (H2SO4) which then begins to react with the infrastructure in a destructive way.
Historically, control of odor and/or corrosion has been implemented through either vapor phase techniques, where the head space of a lift station is treated, or liquid phase techniques, where treatments target the liquid flow. Vapor phase treatments—such as scrubbers—do not provide corrosion control, as opposed to liquid phase techniques, some of which can offer corrosion control.
The most common method of inducing liquid phase treatment, or directly treating the wastewater, inside the collection system, has been by drip dosing chemicals into the systems. A constant and continuous dose of chemical is fed from a large reservoir with a small pump into the collection system, typically at a manhole or pump station. These chemicals are meant to react with the odor causing compounds present in the wastewater or cease their formation and/or release from solution. The main classes of reactions used to control hydrogen sulfide are briefly described in this section.
Oxidation
The chemical oxidation of hydrogen sulfide is accomplished through the use of a compound with a high oxidation potential, called an oxidant, such as hydrogen peroxide or sodium hypochlorite (bleach). This method of treatment involves the direct oxidation of H2S into more benign forms, such as sulfites, sulfates and elemental sulfur. However, it does not affect the presence of SRB, which will likely continue to grow with time. Upon cessation of treatment, expression of hydrogen sulfide can be even worse than before, due to an increased output of the growing SRB layer. In addition, it is likely that increasing quantities of treatment chemical will be required with time for the same reason.
Sulfide Scavengers (Iron Salts)
Chemicals that interact with hydrogen sulfide and sequester, or scavenge, the sulfur into a relatively insoluble form, such as ferric chloride and ferrous chloride, can be used to remove sulfur from the cycle entirely. This treatment has no effect on the presence or production of the SRB layer, and cessation of use will see a return to the emission of hydrogen sulfide, possibly at an elevated level. For this reason, it is likely that increased usage of time will be experienced, as well.
These chemicals will aggressively remove DO from the wastewater; can form scales on iron surfaces, such as pipes and pumps; and can become an ongoing source of sedimentation due to the reaction of iron with sulfur, forming FeS precipitate within the collection system. A continued and/or an elevated risk of corrosion is a significant challenge with the use of ferrous and ferric chloride, due to the generation of acids as part of the scavenging reaction. An example of a reaction in which hydrochloric acid is generated is shown in Equation 5.
(5) 3H2S + 2FeCl3 → S + 2FeS + 6HCl
The bulk scale addition of iron to the collection system also presents the hazard of the contamination of downstream solids. In fact, the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) considers iron salts a persistent environmental hazard [U.S. Peroxide, 2011]. This aspect can significantly add to plant and biosolids processing costs.
pH Adjustment
Because of the manner in which its ions dissociate in the aqueous phase, the release of hydrogen sulfide from wastewater will not occur if the pH is at 9 or above. Through the use of compounds that can induce and maintain significant increases to pH, such as sodium hydroxide or magnesium hydroxide, it is possible to take advantage of this trait and “trap” the sulfide in solution. This can be an expensive process, due to the volume of treatment chemical required to maintain the elevated pH level and the relatively higher expense per gallon (liter).
Also, treatment chemicals can be difficult to keep in solution, especially in colder weather. A good illustration of this is magnesium hydroxide, which has a solubility of only 0.012 grams per liter in water—bordering on the line of insolubility. There is, again, no treatment or reduction of SRB, which can cause the problem to grow with time. Also, once the pH decreases below 9, as can occur downstream of the treatment and/or at the treatment plant, hydrogen sulfide is readily released, possibly at an increased rate due to the increased solution concentration.
Alternate Oxygen Source/Sulfate Substitute
In an anaerobic environment, the microbiology in the collection system will use oxygen from a nitrate (NO3) molecule more readily than from a sulfate (SO4) releasing benign nitrogen rather than hydrogen sulfide. Chemicals—such as calcium or sodium nitrate—are commercially available and can be used for this purpose. These chemicals are expensive, and they feed and grow the SRB layer, potentially requiring a higher volume of treatment over time. Upon cessation of treatment, the expression of hydrogen sulfide can be worse than before.
Excess wet well build-up, requiring increased clean-out cycles due to the addition of the waxes used to stabilize the nitrate molecules, can be encountered downstream. In addition, emerging federal and state regulations are beginning to include nitrate concentrations on discharge limitations. Seldom is there much real time, active monitoring of wastewater sulfide levels, so enough chemical to control peak H2S values is typically added on a constant basis. By treating for peak values with chemicals such as these, excess nitrate will likely be present and actively added to the wastewater, requiring additional denitrification processes or fines, both of which can be expensive.
New Solution—Ozone & Oxygen
An issue with all chemical treatments is that, to introduce them into a collection system, a bulk quantity must be stored nearby. To ensure that chemicals are always available for treatment, continued deliveries to the bulk storage tank must occur. To ensure that the environment is not adversely affected (directly), engineered controls—such as secondary containment and leak monitoring—must be designed, implemented and maintained. Essentially, money is being poured down the drain on a recurring basis with no real solution to the problem taking place.
Ideally, a successful treatment of wastewater odor and corrosion would cease sulfide production, eliminate sulfides that are present, pose no additional hazard to life or the environment, do no harm to the collection system itself and create no additional challenges downstream. In addition, it must be cost-effective. Such a solution is becoming available through the novel approach of introducing ozone and oxygen into collection systems as a means of odor and corrosion control.
Ozone has long been used in water treatment, dating to at least the late 19th century, primarily for the disinfection and the polishing of drinking water [Beltran, 2004]. In Europe, ozone treatment of water is a common process [Lenntech, 1998]. Ozone’s superior environmental sustainability and relative safety versus chemical systems has established it as the favored current and future technology.
The controlled use of ozone as a treatment does not produce any harmful byproducts that could contaminate or harm the environment or ecology. Typically, the only byproducts from its reaction are O2 and inert oxides. Recently, interest in its use in the wastewater industry has led to the development of new and sustainable (green) technology for the treatment of odor and corrosion in wastewater collection systems.
Ozone is a special, naturally-occurring form of atmospheric oxygen. Instead of two oxygen atoms it has three, represented by its chemical formula O3. This third oxygen atom makes it a highly reactive molecule with a high oxidation potential. It has the highest oxidation potential of any commercially available molecule and fourth highest overall with an oxidation potential of 2.07 V. Above it, in oxidation potential, are atomic fluorine (F•, 2.87 V), the hydroxyl radical (•OH, 2.86 V) and atomic oxygen (O•, 2.42 V). Ozone can be generated by exciting a flow of oxygen with sufficient electrical or optical energy. This causes a certain amount of oxygen atoms to split and recombine with other O2 molecules nearby. This is shown in Equation 6.
(6) 3O2 + Energy → 2O3
Under typical treatment conditions, using a relatively pure oxygen stream and a corona discharge chamber, which uses a high voltage electrical arc, this reaction can produce up to 9 to 12 weight percent (wt%) ozone [Drago, 2010], although typically output is in 1 to 9 wt% ozone [PTI, 2011]. The remainder of the stream is left as oxygen. The concentration is limited to this range because of the reaction shown in Equation 7.
(7) 2O3 → 3O2
As ozone amounts rise above this concentration, the destruction reaction (7) becomes more frequent, therefore returning greater quantities of O2 and maintaining this equilibrium. This instability is also why ozone cannot be stored and must be generated immediately prior to application.
Because of its extreme instability and high oxidation potential, ozone is powerful and indiscriminate in its reactions with other chemical species. Ozone has been shown as an effective treatment for the destruction of volatile organic compounds (VOCs), removal of metals, total suspended solids, organic carbon, significant reductions to chemical oxygen demand (COD) and many more. In freshwater, the half-life of ozone is typically 10 to 20 minutes. In wastewater, ozone has been documented as being entirely consumed within 8.6 seconds [Terry, 2010]. This is due to the extreme amount of potential reactants that are present in wastewater—foremost of which, for the purpose of this article, is hydrogen sulfide. The simple structure of hydrogen sulfide makes it an easy target for oxidation by ozone.
In addition to its high oxidation, ozone’s structure tends to create free radicals—chemical species that have unbonded electrons making them highly reactive, especially in water. Not only is the benefit of ozone’s direct reaction with different chemical species realized, but as part of these reactions, additional free radicals can form, which can be more reactive than ozone. These new radicals tend to create more radicals as they react, causing a free radical chain reaction, referred to as the indirect effects of ozone [Beltran, 2004].
Since the source of ozone generation is ambient air, it is the ultimate, sustainable green chemical treatment. The current technology for producing ozone has benefitted from more than 45 years of ongoing development, resulting in cost-effective and robust operation. Using only an oxygen separator, a corona discharge chamber and some compressors and other electrical components, onsite generation of ozone is simple and safe today. This is in sharp contrast to the majority of other current, commercially-available treatments.
Due to the ozone production method, oxygen will also be part of the treatment gas cocktail, which is beneficial because oxygen is also an oxidizer. With an oxidation potential of 1.23 V, oxygen reacts slower than ozone but is an excellent complement. Aside from assisting in oxidation, its primary benefit is increasing the dissolved oxygen (DO) concentration of the wastewater, encouraging the growth of aerobic bacteria. These bacteria are not odorous, corrosive or harmful to collection systems. DO also eliminates the ability of SRB to produce sulfides, either by removing the SRB or promoting the growth of aerobic species over the top that will oxidize any sulfides before they enter the wastewater stream [EPA, 1985].
In terms of a robust and green method for treating and preventing odor and corrosion, the oxygen and ozone combination is at the top of the list. Oxygen is widely available, roughly 21 percent of the atmosphere, and easily converted to ozone. The generation and infusion of these two gases into wastewater collection systems is proven to be clean, safe and cost-effective.
The first method of action is the powerful destructive effects of ozone on hydrogen sulfide, quickly converting it to sulfites and sulfates on contact. In addition, ozone’s antimicrobial properties can help reduce SRB and other microorganisms on pipe walls. As a product of its reaction, oxygen is generated. This adds more oxygen to the treatment gas cocktail, which provides secondary treatment by increasing DO, and allows for more complete use of infused treatment gases.
Because of ozone’s indiscriminate and powerful oxidizing characteristics, concern is sometimes raised that ozone may attack the wastewater infrastructure itself. This is unlikely, especially in wastewater where the liquid phase infusion is implemented. This is due to the high ratio of liquid volume compared to pipe surface area per unit pipe length and the availability of reactants in the liquid portion. It is unlikely that ozone will survive long enough to affect the wastewater infrastructure.
In Part 2 (July), readers will be given the details of a specific treatment process. It includes collected data and analysis. Of interest in this part is the extremes of both temperature and sulfide concentrations under which this treatment study was performed.
Part 3 (August) provides the results of the treatment. Part 4 (September) delivers conclusions and further discussion, supported by data that show how this technology provides savings from chemical use and performance and reliability improvements.
References:
- American Society of Civil Engineers, “ASCE’s Infrastructure Report Card,” ASCE, 2009, http://www.infrastructurereportcard.org/.
- A. Matthews et al, “Control of Hydrogen Sulfide Buildup in Forcemains using Ozone and Oxygen,” Proceedings of the Water Environment Federatin, WEFTEC 2010: Session 101 through Session 112, pp. 7591-7611(21), 2010.
- U.S. Environmental Protection Agency, “Odor and Corrosion Control in Sanitary Sewerage Systems and Treatment Plants,” Design Manual, EPA/625/1-85/018, Cincinnati, OH, 1985.
- U.S. Peroxide, “Iron Salts – Ferric and Ferrous,” 2011, www.h2o2.com
- Beltran, F.J., “Ozone Reaction Kinetics for Water and Wastewater Systems,” Lewis Publishers, 2004.
- Lenntech, “Water Disinfection Application Standards (For EU),” 1998, www.lenntech.com.
- Drago, J.A. et al, “Municipal Wastewater Ozonation Practice in the United States: Past, Present and Future,” Ozone: Science & Engineering, Volume 32, Issue 1, pp. 43-55, 2010.
- Plasma Technics, Inc, “Plasma Block Product Line, Product Detail,” 2011, www.plasmatechnics.com.
- Terry, P.A., “Application of Ozone and Oxygen to Reduce Chemical Oxygen Demand and Hydrogen Sulfide from a Recovered Paper Processing Plant,” International Journal of Chemical Engineering, Volume 2010, Article ID 250235, 2010.


